Pivot-TR
A new class of minimally invasive intervention designed to restore coaptation through dynamic, self-centering support.
Our flow-aligned valve support for
tricuspid regurgitation (TR).

Permanent & Temporary Implant:
Pivot Extend® & Pivot Bridge®
The Pivot-TR platform offers two options that give physicians flexibility to customize care based on patient condition and long-term treatment goals — expanding therapeutic possibilities even for patients once considered too high-risk for intervention.
Pivot Extend® provides a permanent solution.
Pivot Bridge® is a temporary implant that stabilizes frail or high-risk patients, improving valve function and serving as a bridge and diagnostic tool for future therapies.

Minimally Invasive:
Transcatheter / Removable
Pivot-TR is delivered through a transfemoral approach using standard catheter techniques, avoiding the need for open-heart surgery.
The system features non-fixated, or atraumatic anchoring — securing the device without sutures, clips, or tissue penetration.
This design enables easy retrieval via the same tansfemoral approach, offering a reversible therapeutic option if patient conditions or treatment strategies change.
Anchoring:
Non-Fixated Yet Stable
Pivot-TR’s innovative atraumatic anchoring system ensures stable support without tissue fixation. The device is secured across the inferior vena cava and pulmonary artery using non-penetrating anchors, preventing vertical migration along the blood flow axis and keeping the spacer properly seated within the tricuspid valve.

Pulmonary Artery Anchor
A non-fixated anchor positioned within the pulmonary artery that maintains the spacer’s oblique orientation across the tricuspid valve.

Pivot Bridge®
Spiral IVC Anchor
Spiral-shaped anchor positioned within the IVC prevents vertical migration along the circulatory flow axis.

Pivot-TR’s oblique positioning and flow-guided design allow the spacer to self-center dynamically — maintaining optimal valve support even as the heart undergoes natural remodeling and geometric changes over time. This unique balance of stability and adaptability sets Pivot-TR apart from conventional rigid implants.
Adaptability:
Fluid Dynamic
Self-Centering
Pivot Extend®
With the heart, not against it.
By working with the heart’s natural rhythm, and not against it, coaptation is optimized without compromising the heart’s anatomical integrity. The Pivot-TR system’s fluid-dynamic design restores valve function gently and physiologically.
Pivot-TR, Two Solutions:
Pivot Extend® & Pivot Bridge®
Intended Use
Target Disease
TR Severity Treated

Pivot Extend®

Pivot Bridge®
Permanent, definitive therapy
Pre-hab, bridge therapy
Secondary TR
Secondary TR
Severe to torrential
(massive to torrential preferred)
Massive & torrential inclusive
Target Population
Implant Duration
Deployment Method
Patients requiring long-term support
Permanent
Patients needing stabilization
Temporary (~1 week)
Transfemoral venous access /
transcatheter
Transfemoral venous access /
transcatheter
Removal
Anchoring
Spacer Design
Intended for long-term implantation; early retrieval possible if needed
Non-fixated (IVC and PA anchors)
Oblong nitinol mesh encapsulated in a saline-filled balloon
Designed for retrieval after stabilization
Non-fixated (IVC and PA anchors)
Oblong nitinol mesh with ePTFE sheath and flow windows
Spacer Materials
Valve Interaction
Adaptability
Customization
Nitinol mesh, saline-filled balloon
Nitinol mesh, ePTFE sheath
Supports leaflet coaptation without fixation
Supports leaflet coaptation without fixation
Self-centering and dynamic with heart remodeling
Self-centering and dynamic with heart remodeling
Balloon set during implantation
(12mm~20mm dia.)
Spacer size set pre-implantation
(9mm, 12mm,15mm, 18mm dia.)
Pivot Extend® and Pivot Bridge® are two therapies built on the Pivot-TR platform, designed to treat tricuspid regurgitation, including massive and torrential cases, using a minimally invasive, flow-adaptive approach.
Pivot Extend® is a permanent solution with an adjustable saline-filled spacer, offering long-term therapy for patients ineligible for surgery. Pivot Bridge® is a temporary implant that stabilizes high-risk or frail patients before surgery or further intervention with potential application as a diagnostic tool.
Both devices use a non-fixated, self-centering spacer and are tailored to match individual patient needs.
For more information, contact us at INFO@tau-medical.com.
Procedure:
Pivot Extend®
Clinical Evidence
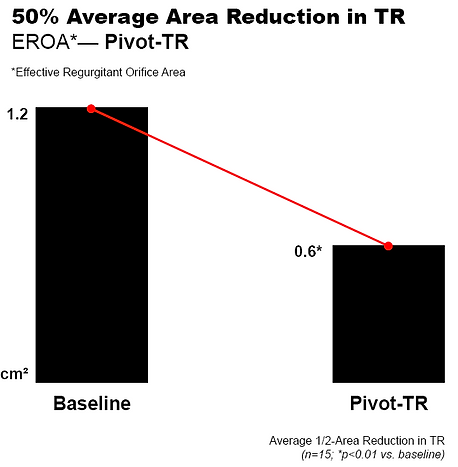
Pivot-TR has demonstrated strong first-in-human results, achieving significant TR reduction, right heart remodeling, and symptom improvement in high-risk patients with severe secondary TR. Building on these outcomes, Tau Medical is expanding clinical trials and compassionate-use programs for Pivot Extend® and Pivot Bridge® across multiple sites in
South Korea, Germany, Georgia, Japan, and India.

Post Procedure
TR Grade Reduction
1 Grade Reduction
2 Grade Reduction
3 Grade Reduction
Anatomical Stabilization

8.1% Avg. RV Volume Reduction

10.2% Avg. TV Annulus Reduction
Clinical Stabilization

•NYHA Class Improvement (NYHA 2.5 -> 1.5, p<0.05)
•Body Weight : 1.5 Kg ↓
•eGFR : 10%↑ (p<0.05)
Study Overview
Study Type
Centers
Sample Size
Patient Profile
Implant Duration
Analysis
Multi-center, single-arm, short-term implantation
11 hospitals across South Korea
15 patients (out of 19 screened)
Severe symptomatic secondary TR
≤ 7 days (temporary implantation)
Core lab-reviewed echocardiography and cardiac CT
Learn more about Tau Medical and our full innovation pipeline at tau-medical.com.
Commonly Asked Questions
What is Pivot-TR and how does it differ from existing transcatheter tricuspid therapies?
Pivot-TR is a spacer-based therapy that is dynamically anchored without leaflet or annular fixation. Unlike rigid or fixated implants, it is designed to adapt to the patient’s anatomy and valve motion, offering a less invasive and repositionable option for tricuspid regurgitation (TR).
What is the difference between Pivot Bridge® and Pivot Extend®?
Pivot Bridge® is a temporary implant intended for short-term stabilization of tricuspid regurgitation (TR), either during clinical evaluation or as a bridge to definitive therapy. The specific design of the Pivot Bridge® spacer with flow windows allows for ease of catheter-removal without the need for spacer deflation. Pivot Extend® is a permanent device designed for longer-term use. Both systems feature the same flow-guided, dynamically anchored design, with an obliquely positioned spacer to improve valve coaptation—without sutures or annular fixation.
What clinical need does Pivot-TR address?
Pivot-TR is designed for patients with severe tricuspid regurgitation (TR) who are poor candidates for surgery or are not adequately served by current transcatheter options. It offers a novel spacer-based solution that is dynamically anchored and obliquely positioned to optimize valve coaptation.
How is Pivot-TR implanted?
Pivot-TR devices are implanted via transfemoral venous access. Pivot-TR's unique design allows for flow-guided positioning and self-centering within the tricuspid orifice, reducing reliance on complex imaging or fixation mechanisms.
How is the device anchored without fixation to the leaflets or annulus?
Pivot-TR is dynamically stabilized through two natural bends along the venous axis—from the IVC to the tricuspid valve, and from the tricuspid to the pulmonary artery. This structure provides secure positioning without tissue fixation.
Can the device be repositioned or removed?
Yes. Pivot-TR is designed to be both retrievable and repositionable during the procedure, offering greater procedural flexibility and enhanced safety. Pivot Bridge®, in particular, is a temporary implant engineered for complete removal via a transcatheter approach.
Can Pivot Extend® be removed if needed?
Yes. Pivot Extend® is designed as a permanent implant. It may be removable under specific clinical conditions, though it is intended to remain in place long-term to support sustained therapeutic benefit.
What imaging modalities are required for implantation?
Standard fluoroscopy and TTE or TEE are used for guidance. The simplicity of the procedure may reduce reliance on advanced imaging relative to other TR therapies.
What are the regulatory and development milestones for Pivot-TR?
Pivot-TR has achieved early clinical use under compassionate or early feasibility protocols. Regulatory pathways are underway in multiple regions, with ISO 13485 certification in progress and KFDA/FDA/CE/India strategies being evaluated.
Who can I contact for partnership opportunities?
You can reach out directly via our contact form to explore strategic collaboration, compassionate use (expanded access) opportunitites, or for more information.





